

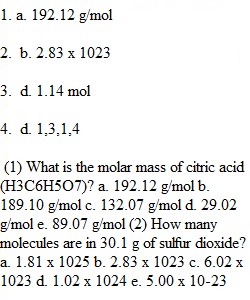
Q CHM 1032 Chemical Quantities and Reactions Focus Concepts Name____________________________ Date______________ Directions: show all mathematical work, using dimensional analysis and select the correct answer. (1) What is the molar mass of citric acid (H3C6H5O7)? a. 192.12 g/mol b. 189.10 g/mol c. 132.07 g/mol d. 29.02 g/mol e. 89.07 g/mol (2) How many molecules are in 30.1 g of sulfur dioxide? a. 1.81 x 1025 b. 2.83 x 1023 c. 6.02 x 1023 d. 1.02 x 1024 e. 5.00 x 10-23 (3) How many moles of hydrogen are there in 6.50g of ammonia (NH3)? a. 0.382 mol b. 1.39 mol c. 0.215 mol d. 1.14 mol e. 2.66 mol (4) What are the stoichiometric coefficients in the following equation when it is balanced? SiCl4(s) + H2O(l) ? H2SiO3(s) + HCl(g) a. 1,3,1,3 b. 1,1,3,4 c. 1,4,1,4 d. 1,3,1,4 e. 3,9,1,4 (5) Ammonia is produced by the reaction of nitrogen and hydrogen: N2(g) + H2(g) ? NH3(g) (a) Balance the chemical equation. (b) Calculate the mass of ammonia produced when 35.0g of nitrogen reacts with hydrogen.
View Related Questions